Michigan chestnut crop report – July 24, 2025
Japanese beetle and rose chafer adults are out and active. Symptoms of wilt are being reported in some orchards.
Weekly weather review
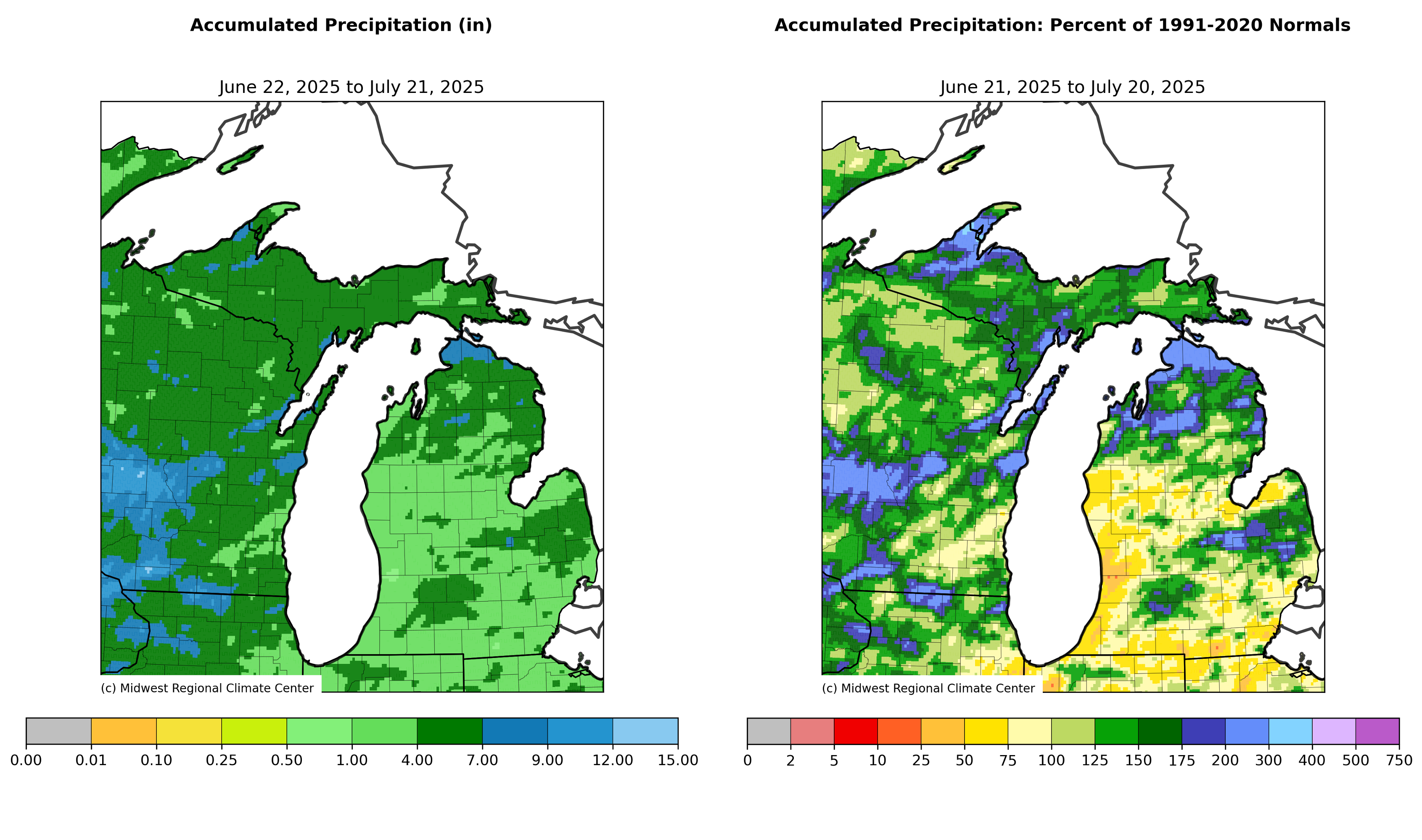
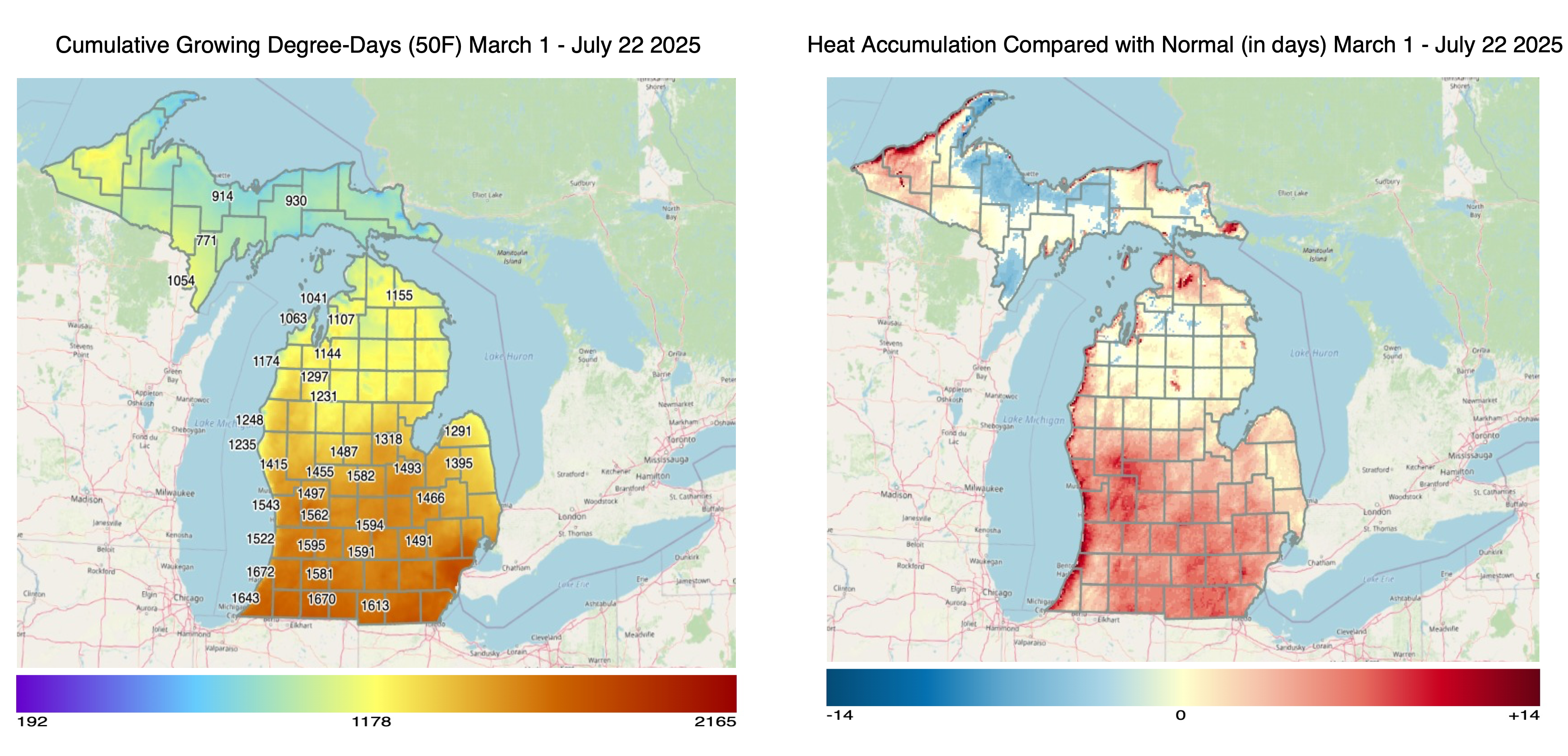
Temperatures across much of Michigan have been above average over the last month and very hot Wednesday-Thursday, July 23-24. With the warm weather, growing degree days (GDD) base 50 degrees Fahrenheit are now above average for most areas of the Lower Peninsula (March 1-July 22). The Upper Peninsula is more variable with below average to above average heat accumulation depending on location. Accumulated precipitation has been above average across most of the Upper Peninsula and northern lower Michigan and below average across west central, southwest and southcentral Michigan.
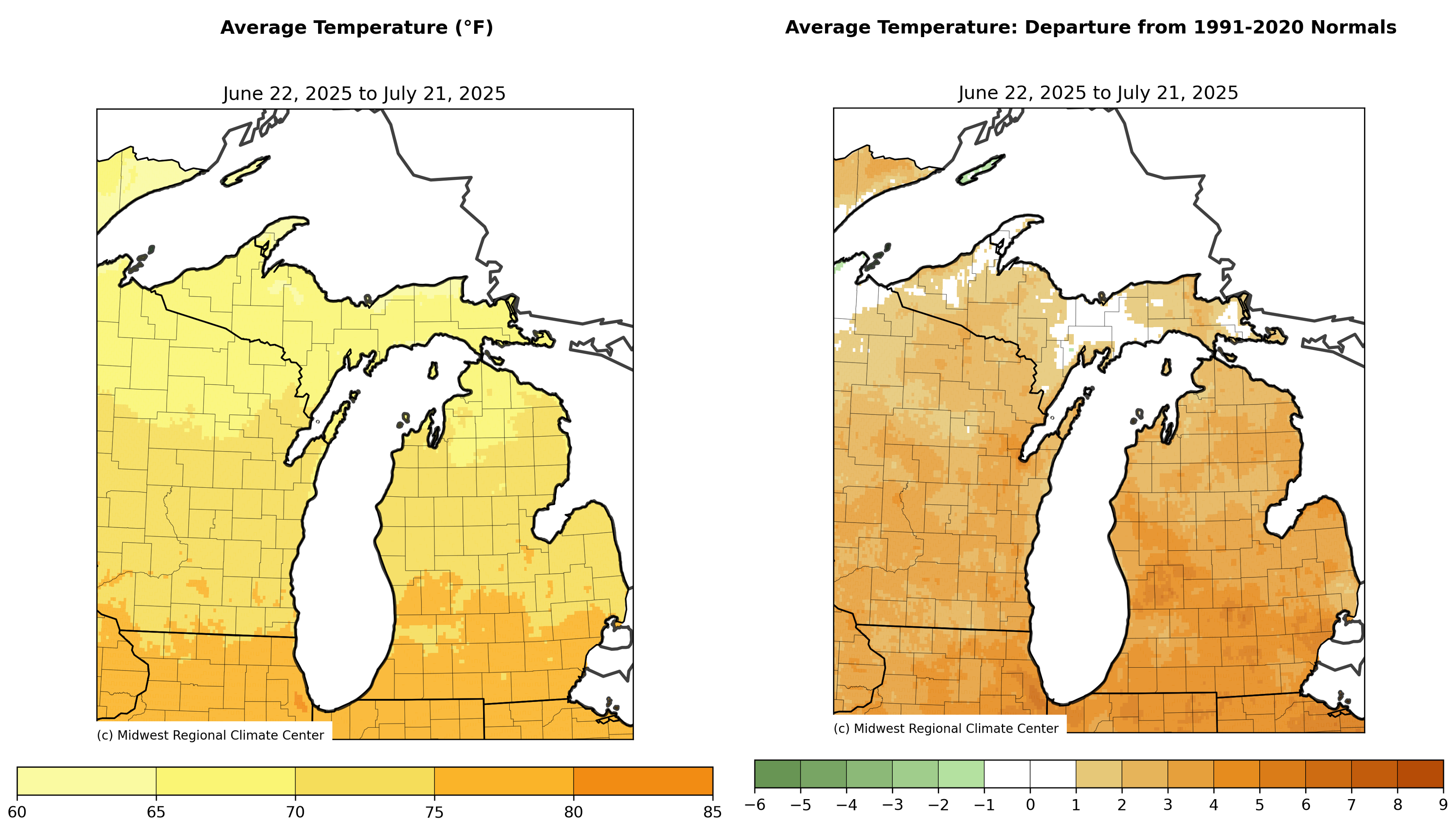
Accumulated GDD base 50 range from near 800 in the Upper Peninsula and around 1,700 in certain areas of southern Michigan. GDD base 50 are near or just slightly below the long-term average across most of state.
Looking ahead
Scattered showers and thunderstorms are possible across much of the state Thursday afternoon (July 24) through the evening. There is potential for additional precipitation across southern Michigan through the weekend.
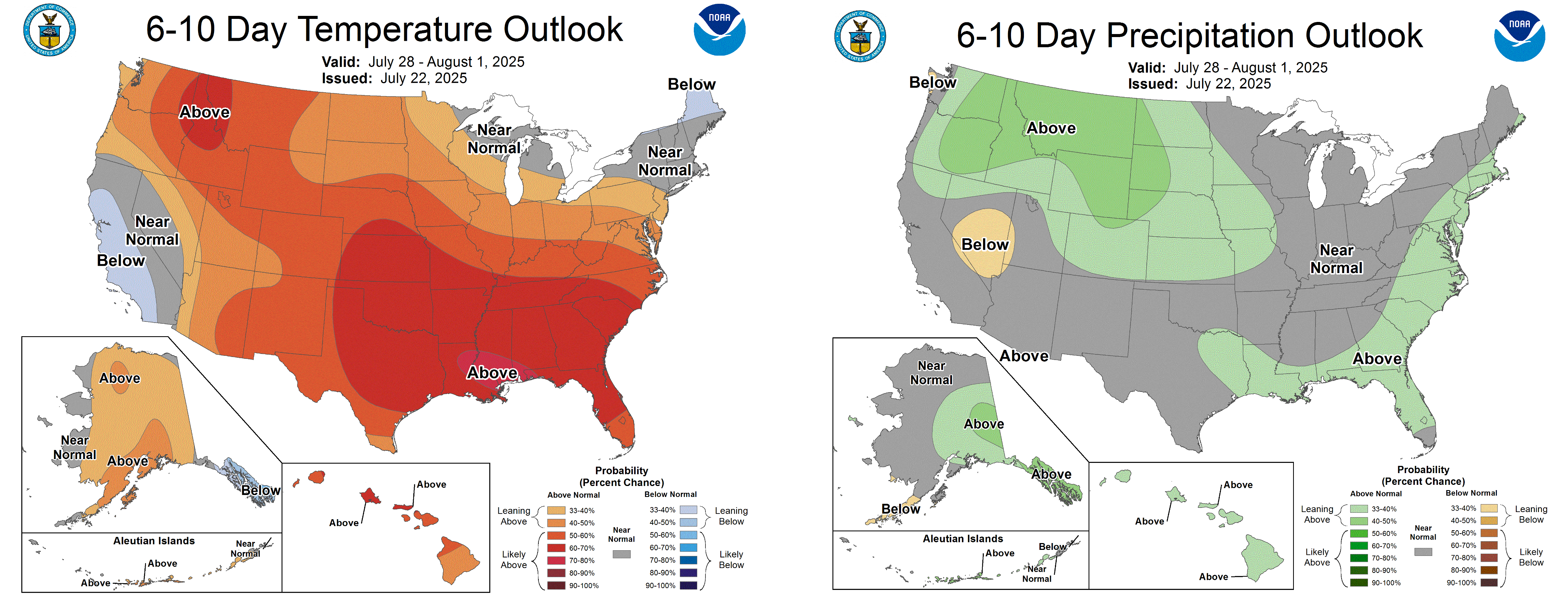
As the heat dome gives way, high temperatures will drop to the low 80s in northern Michigan and high 80s and further south. Lows will be in the 60s-70s through the weekend and into early next week. From July 23-30, the forecast suggests 1-2 inches of precipitation depending on location. The 6-10 day forecast suggests slightly warmer than normal temperatures and near normal precipitation.
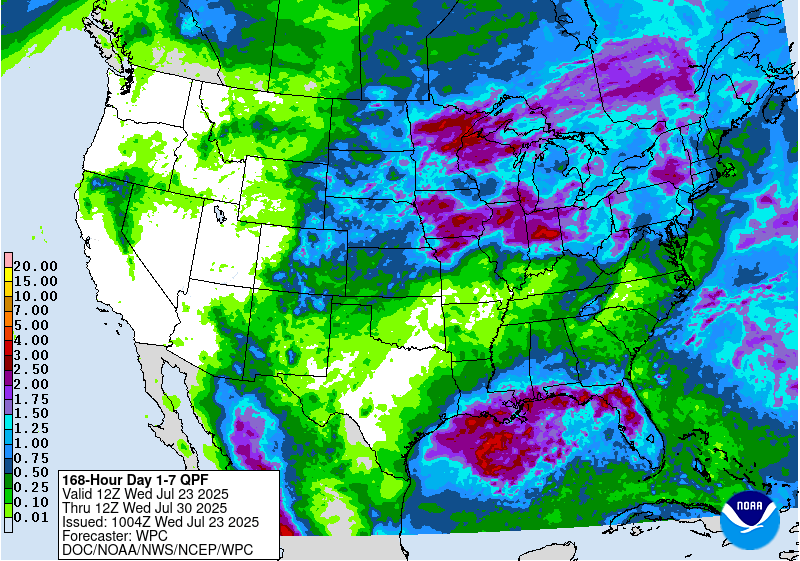
For the most recent Michigan State University agriculture weather forecast, visit: MSU Extension Agriculture Weather.
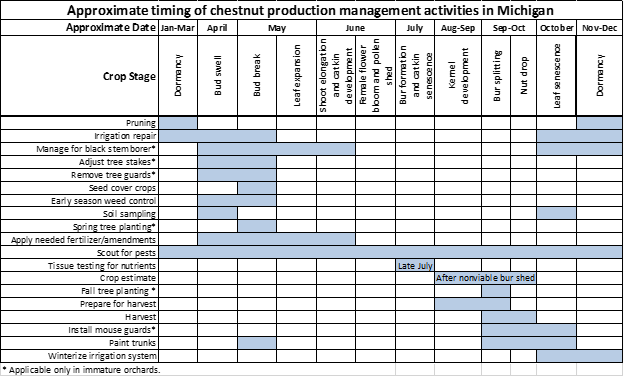
Management activities
Growers should focus on scouting for pests.
Fertility
Most growers using granular fertilizers have applied them. Be ready to pull leaf plus petiole nutrient samples in late July.
- Collect samples from this year’s new growth before vegetative terminal growth stops for the year. Terminal growth typically stops in early August as the trees start developing burs. Samples taken after terminal growth stops will not be accurate.
- Collect five to 10 leaves per tree from multiple terminal branches. Sample along the length of the new growth- do not just select the outermost leaves.
- Carefully read the protocol from the testing facility you plan to utilize to ensure you receive a valid report.
For comparison's sake, it is important to pull samples at the same time of year. For more specific information on pulling leaf samples, see the Michigan State University Extension article, “MSU seeking assistance in establishing baseline nutrient data for chestnuts.”
Leaf and petiole nutrient labs:
- Agro-Liquid, St. Johns, MI
- A&L Great Lakes, Ft. Wayne, IN
- New Age Laboratories, South Haven, MI
- Brookside Labs, New Knoxville, OH
SAP analysis labs:
- Agro-K, Minneapolis, MN
- Crop Health Labs, Bellville, OH
- New Age Laboratories, South Haven, MI
For nutrient management considerations, reference the Michigan Chestnut Management Guide or the Nutrient Management section of the MSU Extension Chestnut website. To receive nutrient management recommendations from MSU, pick up a commercial test at your local MSU Extension office.
Integrated pest management
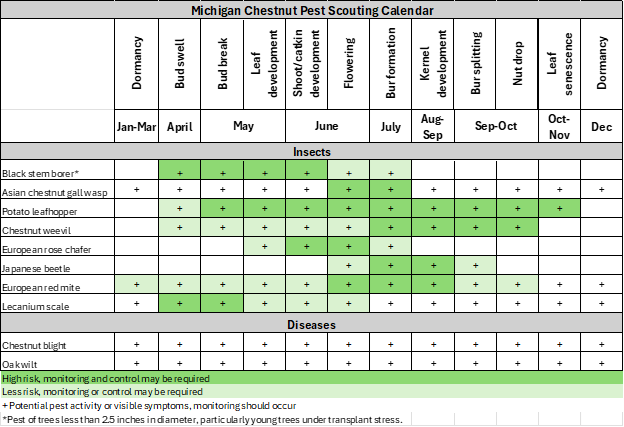
Review the Michigan Chestnut Management Guide and the pest management section of the chestnut webpage for additional pictures and pest management information.
Potato leafhopper
Potato leafhopper numbers are modest in most areas. Like many plants, chestnuts are sensitive to the saliva of potato leafhopper, which is injected by the insect while feeding. Damage to leaf tissue can cause reduced photosynthesis which can impact production and quality and damage the tree. Most injury occurs on new tissue on shoot terminals with potato leafhopper feeding near the edges of the leaves using piercing-sucking mouthparts. Symptoms of feeding appear as whitish dots arranged in triangular shapes near the edges. Heavily damaged leaves are cupped with brown and yellowed edges and eventually drop from the tree. Severely infested shoots produce small, bunched leaves with reduced photosynthetic capacity.
Adult leafhoppers are pale to bright green and about 1/8 inch long. Adults are easily noticeable, jumping, flying or running when agitated. The nymphs (immature leafhoppers) are pale green and have no wings but are very similar in form to adults. The potato leafhopper can’t survive Michigan’s winter and survives in the Gulf States until adults migrate north in the spring on storm systems.
Scouting for potato leafhopper should be performed weekly as soon as leaf tissue is present to ensure early detection. More frequent spot checks should be done following rainstorms, which carry the first populations north. For every acre of orchard, select five trees to examine and inspect the leaves on three shoots per tree (15 shoots per acre). The easiest way to observe potato leafhopper is by flipping the shoots or leaves over and looking for adults and nymphs on the underside of leaves. Pay special attention to succulent new leaves on the end of branches. For more information on insecticides available for the treatment of potato leafhopper, refer to the Michigan Chestnut Management Guide
Mites
Be on the lookout for European red mites. European red mites overwinter as eggs in bark crevices and bud scales and are the most common mite species in Michigan chestnut orchards. Eggs are small spheres, about the size of the head of a pin with a single stipe or hair that protrudes from the top (this is not always visible). Eggs can be viewed with a hand lens or the naked eye once you have established what you are looking for.
Scout for overwintering eggs and early nymph activity in the spring to assess population levels in the coming season. As temperatures warm, overwintering eggs hatch and nymphs move onto the emerging leaves and start feeding. Adult European red mites are red in color and have hairs that give them a spikey appearance. Adult and nymph feeding occurs primarily on the upper surface of the leaves. This first generation is the slowest of the season to mature and reproduce, typically taking a full three weeks. This slow development is due to the direct link between temperature and mite development. Summer generations, favored by the hot and dry weather, can complete their lifecycles much faster with as little as 10 days between generations under ideal conditions.
As you scout, remember that not all mites are bad. Consider documenting the levels of predacious mites in your orchard. If healthy populations of mite predators exist, they will continue to feed on plant parasitic eggs and nymphs and can be an effective component of your mite management program. Predaceous mites are smaller than adult European red mites and twospotted spider mites, but they can be seen with a hand lens and typically move very quickly across leaf surfaces.
Mite control starts with monitoring early in the spring looking for the overwintering eggs (European red mites) and assessing the mite pressure. Ideally, growers will be using limited insecticides with miticidal activity in their season long programs, as that protects their beneficial mite populations which help minimize pest mites. If pest mite populations are high enough to require control, superior oil application when the trees are dormant is an effective method of treatment. If issues with mites arise during the growing season, refer to the Michigan Chestnut Management Guide for control options.
Asian chestnut gall wasp
Growers with Asian chestnut gall wasp populations in the orchard and those adjacent to known infestations should continue scouting as we are in the degree-day window for adult flight. Chestnut growers in counties west of Highway 127, especially areas south of I-96 in lower Michigan, should be scouting their trees for evidence of Asian chestnut gall wasp during the growing season and again in fall or winter, after leaf drop.
The Asian chestnut gall wasp produces one generation per year and is a parthenogenetic species, meaning that all wasps are female and reproduction occurs asexually. Thus, even a single wasp can start a new infestation. Gall wasp adults are very small, about 1/8 inch (3 mm) long, and have black bodies with yellow legs. Adult wasps lay eggs inside chestnut buds over a four- to six-week period, typically from the last week of June to the second week in August in southwest Michigan. This period corresponds to approximately 1,050 to 2,100 growing degree days (GDD) base 50 F. Eggs hatch after about 40 days. The tiny larvae, which cannot be seen without magnification, remain dormant in the buds throughout the winter until the following spring.
In late spring and summer, green or reddish galls can be observed on branches or leaves. In fall and winter, look for dried, brown galls on the shoots. Many old galls remain on the tree through winter and are more visible after leaves drop in fall. These old galls can remain attached to the trees for at least one or two years after the wasps have emerged. In Michigan, we have a parasitic wasp called Torymus sinensis which helps naturally suppress the gall wasp and uses the galls to overwinter. For this reason, do not remove the current season’s galls from trees to support this beneficial wasp.
For those with young trees, carefully monitor for Asian chestnut gall wasp adult flight and consider applying a pyrethroid insecticide for control. Young trees are particularly susceptible to severe damage by Asian chestnut gall wasp due to the limited number of buds and the need to establish tree structure during the first years of establishment. Hang yellow sticky traps in mid-late June in the canopy to passively trap for adult flight and better time pesticide applications. Growers with mature trees should monitor the impact of Asian chestnut gall wasp on yield and tree health and make management decisions accordingly.
For more information, check out the Asian Chestnut Gall Wasp bulletin.
Rose chafer and Japanese beetle
Rose chafers and Japanese beetle are considered generalist pests and affect many crops, particularly those found on or near sandy soils or grassy areas. The adult beetles feed heavily on foliage and blossom parts of numerous horticultural crops in Michigan and can cause significant damage to chestnut orchards. Rose chafer and Japanese beetle adults are currently active in all chestnut producing regions of the state.
These beetles can be particularly damaging on young trees with limited leaf area. Visual observation while walking a transect is the best method for locating them. Because of their aggregating behavior, they tend to be found in larger groups and are typically relatively easy to spot.
There are no established treatment thresholds or data on how much beetle feeding damage a healthy chestnut tree can sustain, but well-established and vigorous orchards will likely not require control. Young orchards with limited leaf area will need to be managed aggressively. For more information on insecticides available for the treatment of rose chafer, refer to the Michigan Chestnut Management Guide.
Brown rot
Growers in most locations have completed their bloom-time applications for chestnut brown rot management. Chestnut rots pose a serious threat to the global production of edible chestnuts, significantly affecting their quality and marketability. In Michigan, various pathogens contribute to nut rots, but in recent years brown rot, caused by the fungus Gnomoniopsis smithogilvyi, has become the leading cause of nut decay. G. smithogilvyi was first detected in Michigan in 2016. In a 2017 survey of orchards, 80% tested positive for the pathogen. Since that time, the incidence of brown rot has steadily increased with as much as 9% of the chestnuts processed by the largest cooperative in Michigan showing symptoms of brown rot at harvest. The disease is characterized by a gradual browning of the kernel that develops in the field or after harvest.
As the prevalence of this disease has increased around the world, researchers have been studying its life cycle and disease cycle to understand how and when the pathogen infects chestnuts. There are two types of infections in the disease cycle. The primary infection is caused by sexual spores that overwinter protected by a reproductive structure on dead plant material on the orchard floor. In spring and summer, these sexual spores are released from the understory and create secondary infections that develop asexual spores which are spread by rain splash, wind and insects and cause new infections in a repeating cycle during the growing season. In the fall, cool temperatures again trigger the development of overwintering spores, which then persist into the following growing season and start the cycle all over again.
The spores from the secondary infection cycle can infect flowers, leaves and branches. It is also important to note that the fungus can exist as an endophyte, not causing disease or symptoms—simply surviving on plant parts and debris. However, these endophytic populations become pathogenic when optimal conditions and opportunities for infection occur. When conducive temperature, rainfall and humidity levels coincide with female flower bloom, spores can move to and germinate on female flower parts, entering the plant through the flower and establishing infection in the developing nut embryo.
New research out of Michigan State University has provided new insight into chestnut brown rot management. Growers planning to manage brown rot this season should carefully review “Chestnut brown rot management in the field” in preparation for a bloom-time preventative fungicide program.
Oak wilt
Chestnut trees with oak wilt infection may be showing symptoms at this time of year. Oak wilt primarily affects trees in the red oak group (Lobatae). Red oaks typically die within a year of infection. White oaks can be infected but generally do not succumb to the disease. Both oaks and chestnut are members of the family Fagaceae, so it is perhaps not surprising that oak wilt can infect chestnut trees. The following disease cycle is based on research with oaks but may shed some light on the behavior of oak wilt in chestnut.
Oak wilt can spread overland by beetles or underground via root grafting. Overland spread occurs when tiny sap beetles, also known as picnic beetles pick up viable fungal spores from an oak that has recently died from oak wilt, then feed on sap oozing from a wound on a live oak. The wound must be deep enough to penetrate the sapwood.
When beetles move from a tree infected with oak wilt to a wound on a healthy tree, the spores can establish a new infection. The fungus grows into and eventually plugs up the xylem cells (sapwood) the tree uses to transport water. This causes the tree to wilt and die. Oaks infected in the spring can die within a few weeks. Trees infected in the fall may not die until the following year.
Beetles are most likely to be carrying spores in late spring and early summer. Therefore, activities that may wound trees, including pruning, should be avoided from April 15 through July 15 to reduce the risk of overland transmission and new oak wilt infections. Unavoidable or accidental wounds from storms or lawn equipment should be painted with a pruning sealer or latex paint as soon as possible.
Once a tree is infected, below ground transmission of oak wilt from tree-to-tree can occur via root grafting between infected oaks and adjacent healthy trees. Root grafting, a natural phenomenon, occurs when adjacent or nearby trees of the same species form physical connections between their roots, allowing the trees to exchange water, carbon and nutrients. A tree killed by oak wilt can become the center of a disease outbreak, resulting in expanding pockets of dead and dying trees. When oak wilt is detected, management can involve disrupting the connected roots. This can be achieved by excavating deep trenches and removing infected oaks plus a buffer of healthy oaks to curtail disease spread.
Once oak trees are infected with oak wilt, they cannot be saved. Managing an outbreak early and effectively as described above can prevent additional oaks from becoming infected and dying.
Growers may scout for oak wilt within their chestnut orchards and in any adjacent oak stands. Look for dead or declining trees. For reference, you may refer to the Michigan Department of Natural Resources (MIDNR) interactive map highlighting locations where oak wilt has been confirmed in Michigan. This map only shows detections officially reported to the MIDNR and is not a complete listing of all oak wilt locations.
An infected tree is often first noticed when leaves take on a wilted, light green appearance during the summer before progressing to yellow and brown and dropping prematurely. The presence of dark streaking under the bark on branches and between the cambium and bark on trunk cross sections are common symptoms of oak wilt. However, the only way oak wilt can be confirmed is either through observation of a mycelial mat forming under the bark or from laboratory testing in a diagnostic lab.
To date, mycelial mats have only been found on oak trees. Whether they will form on chestnut trees is not yet known. Growers who suspect they may have oak wilt affecting their chestnut tree may send a sample to MSU Plant and Pest Diagnostics following these guidelines.
For more information on oak wilt and chestnuts, review the article MSU investigating oak wilt as the cause of sudden chestnut tree decline. Also see the July 10, 2025 Chestnut Chat for a deeper dive into oak wilt.
Blight
Existing chestnut blight infections (caused by Cryphonectria parasitica) can be observed at this time with orange fruiting bodies visible on cankers. There are no commercially available treatments for chestnut blight. Growers may prune out infected branches or cull whole trees as needed to limit disease pressure. Infested material should be burned or buried to further limit inoculum spread. To learn more about chestnut blight, visit the pest management section of the chestnut webpage.
Stay connected
For more information on chestnut production, visit www.chestnuts.msu.edu. Sign up to receive our newsletter and join us for the 2025 MSU Chestnut Growers Chat Series.
If you are unsure what is causing symptoms in the field, you can submit a sample to MSU Plant & Pest Diagnostics. Visit their webpage for specific information about how to collect, package, ship and image plant samples for diagnosis. If you have any doubt about what or how to collect a good sample, please contact the lab at 517-432-0988 or pestid@msu.edu.
Become a licensed pesticide applicator
All growers utilizing pesticide can benefit from getting their certification, even if not legally required. Understanding pesticides and the associated regulations can help growers protect themselves, others, and the environment. Michigan Pesticide Applicator Certification is administered by the Michigan Department of Agriculture and Rural Development. You can read all about the process by visiting the Pesticide FAQ webpage. Michigan State University offers a number of resources to assist people pursuing their license, including an online study/continuing ed course and study manuals.
This work is supported by the Crop Protection and Pest Management Program [grant no 2024-70006-43569] from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.



 Print
Print Email
Email
